2023 Guideline for Diagnosis and Management of Atrial Fibrillation: Key Perspectives
21 August 2024
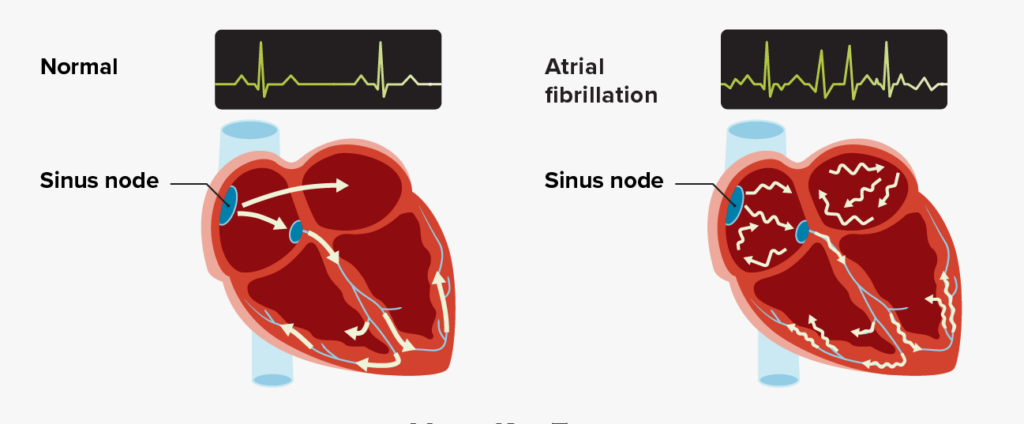
2023 Guideline for Diagnosis and Management of Atrial Fibrillation: Key Perspectives
The following are key perspectives from the 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation (AF):
- The current guideline’s classification of AF focuses on the stages of AF including the pre-detection period. Stage 1: at risk for AF presence of modifiable and nonmodifiable risk factors, Stage 2: pre-AF (evidence of structural or electrical findings predisposing to AF), Stage 3A: paroxysmal AF (intermittent, lasting up to 7 days), Stage 3B: persistent AF (continuous and sustained for over 7 days and requires intervention), Stage 3C: long-standing persistent AF (continuous AF lasting >12 months), Stage 3D: successful AF ablation (free from AF after ablation or surgical intervention), and Stage 4: permanent AF (no further attempts at rhythm control). Early rhythm control is associated with a greater likelihood of maintaining sinus rhythm in the long term and minimizing AF burden and reducing the progression of the disease.
- Lifestyle and risk factor modification is a pillar of AF management to prevent onset, progression, and adverse outcomes. For patients with AF, the guideline recommends: 1) weight loss in those with body mass index >27 kg/m2, 2) moderate-to-vigorous exercise training to a target of 210 minutes per week, 3) tobacco cessation, 4) minimization or elimination of alcohol consumption, 5) optimal blood pressure control, and 6) screening for sleep-disordered breathing.
- The guideline endorses the use of a validated clinical risk score, such as CHA2DS2-VASc, ATRIA, or GARFIELD-AF. Patients with AF at intermediate annual risk of thromboembolic events (<2%) may benefit from consideration of additional factors that might modify their risk of stroke: higher AF burden, persistent or permanent AF versus paroxysmal, obesity, hypertrophic cardiomyopathy, poorly controlled hypertension, estimated glomerular filtration rate (<45 mL/h), proteinuria (>150 mg/24 h), enlarged left atrial volume (≥73 mL) or diameter (≥4.7 cm).
- Bleeding risk scores should not be used in isolation to determine eligibility for oral anticoagulation (OAC) but instead to identify and modify bleeding risk factors and to inform medical decision-making. In patients diagnosed with AF who have an estimated annual risk of stroke or thromboembolic events ≥2%, therapy to reduce the risk of stroke should be based on the risk of thromboembolism, whether the AF pattern is paroxysmal, persistent, long-standing persistent, or permanent. Direct oral anticoagulants are preferred over warfarin except in patients with mitral stenosis or mechanical heart valves. In patients with AF who are candidates for anticoagulation and without an indication for antiplatelet therapy, aspirin either alone or in combination with clopidogrel as an alternative to anticoagulation is not recommended to reduce stroke risk. In patients with AF and chronic coronary artery disease (beyond 1 year after revascularization or coronary artery disease not requiring coronary revascularization) without a history of stent thrombosis, OAC monotherapy is recommended over the combination therapy of OAC and single antiplatelet agent (aspirin or P2Y12 inhibitor) to decrease the risk of major bleeding.
- In patients with symptomatic AF in whom antiarrhythmic drugs have been ineffective, contraindicated, not tolerated, or not preferred, catheter ablation is useful to improve symptoms. In selected patients (generally younger with few comorbidities) with symptomatic paroxysmal AF, catheter ablation is useful as first-line therapy to improve symptoms and reduce progression to persistent AF. In patients (other than younger with few comorbidities) with symptomatic paroxysmal or persistent AF who are being managed with a rhythm-control strategy, catheter ablation as first-line therapy can be useful to improve symptoms. Catheter ablation is recommended in appropriate AF patients with heart failure with reduced ejection fraction.
- In view of recent studies, more prescriptive recommendations are provided for patients with device-detected AF (implantable devices and wearables). For patients with a device-detected atrial high-rate episode (AHRE) lasting ≥24 hours and with a CHA2DS2-VASc score of 2 or equivalent stroke risk, it is reasonable to initiate OAC within a shared decision-making framework that considers episode duration and individual patient risk. For patients with a device-detected AHRE lasting between 5 minutes and 24 hours and with a CHA2DS2-VASc score ≥3 or equivalent stroke risk, it may be reasonable to initiate anticoagulation within a shared decision-making that considers episode duration and individual patient risk. Patients with a device-detected AHRE lasting <5 minutes and without another indication for OAC should not receive OAC.
- The current guideline gives left atrial appendage occlusion (LAAO) devices a higher-level Class of Recommendation. In patients with CHA2DS2-VASc score ≥2 and a contraindication to long-term OAC, percutaneous LAAO (pLAAO) is reasonable (Class 2a). In patients with a moderate to high risk of stroke and a high risk of major bleeding on OAC, pLAAO may be a reasonable alternative to OAC (Class 2b).
- In patients with AF who are identified in the setting of acute medical illness or surgery, outpatient follow-up for thromboembolic risk stratification and decision-making on OAC, as well as AF surveillance, can be beneficial given a high risk of AF recurrence. In patients with AF who are identified in the setting of critical illness due to sepsis, the benefits of anticoagulation during critical illness for stroke prevention are uncertain.